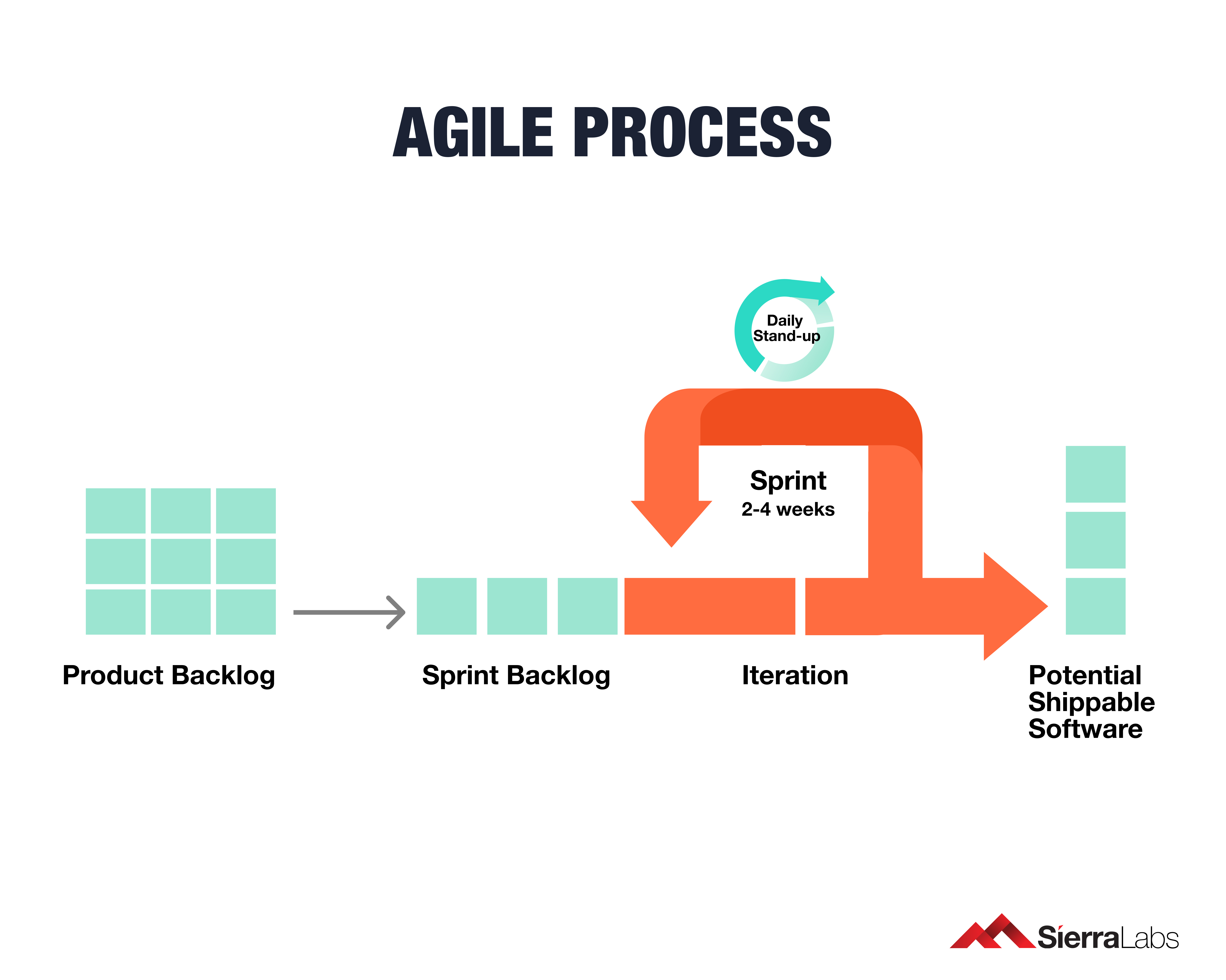
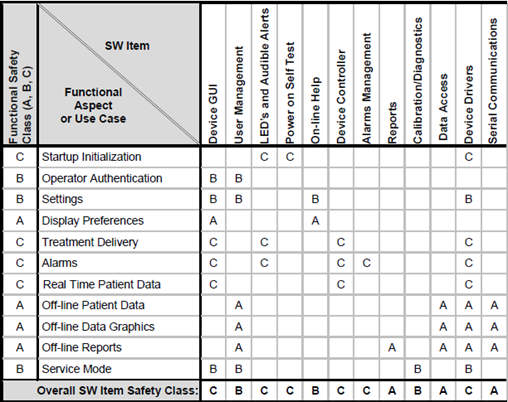
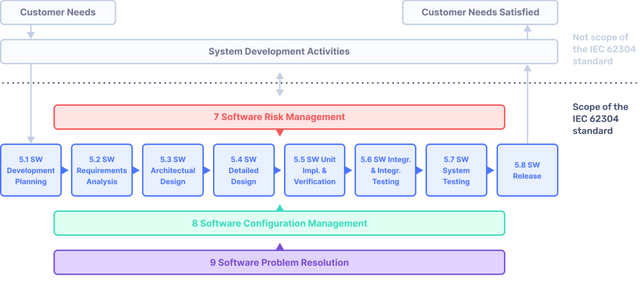
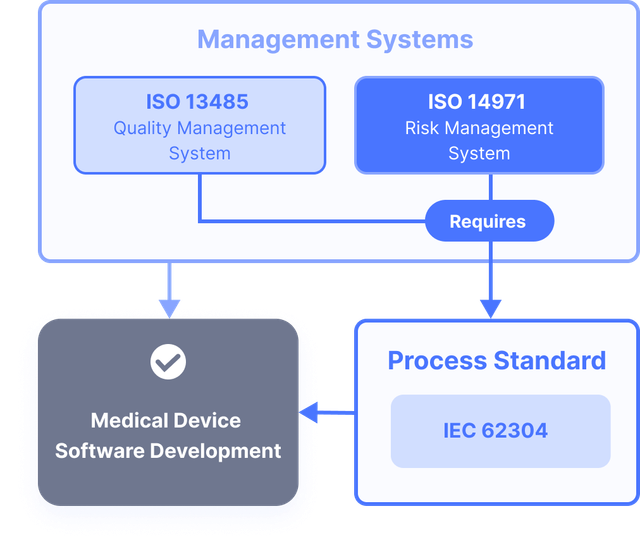
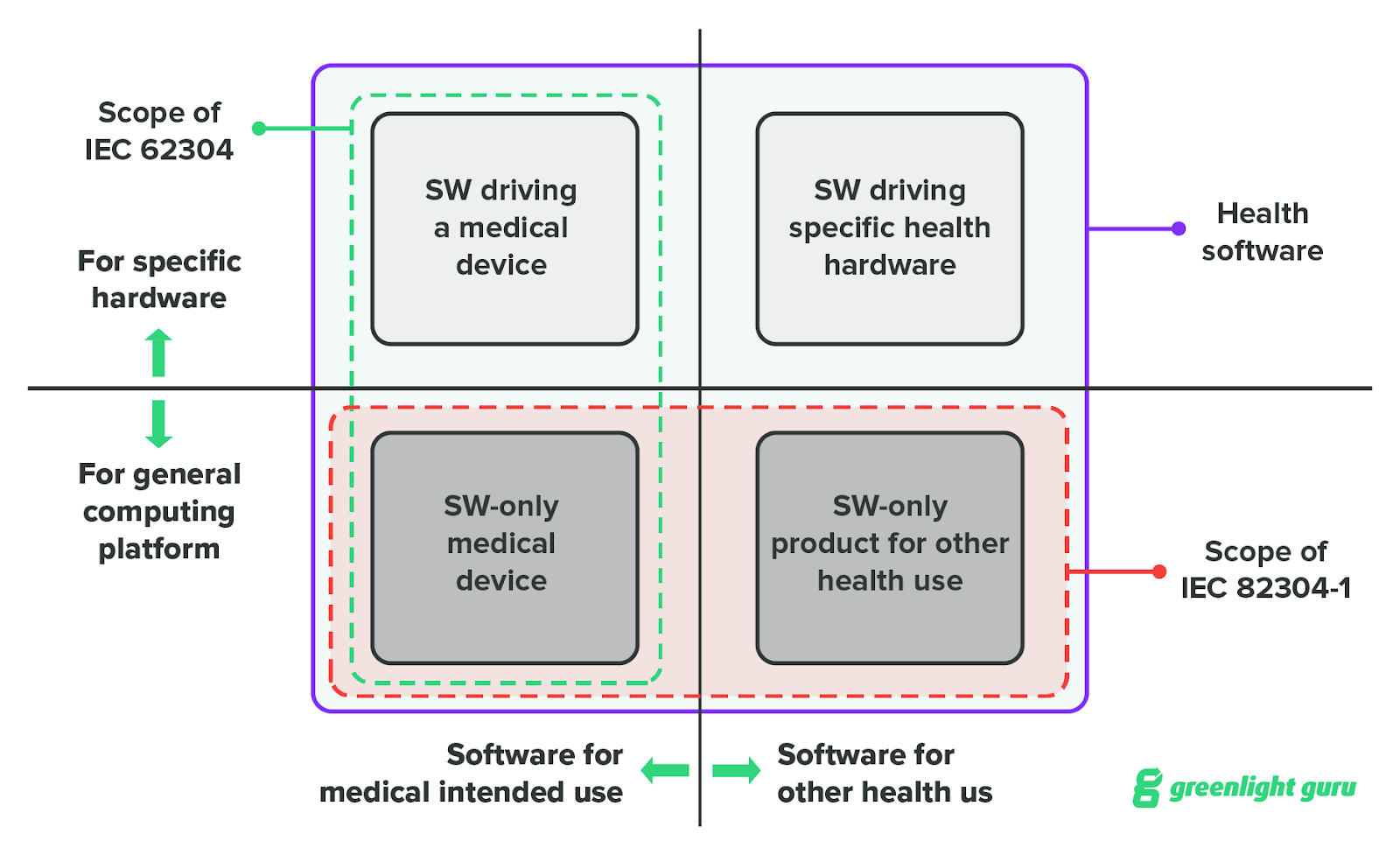
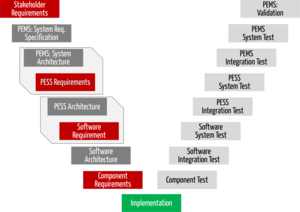
IEC 62304: Medical Device Software LifeCycle Processes | by Dr Stephen Odaibo | The Blog of RETINA-AI Health, Inc. | Medium
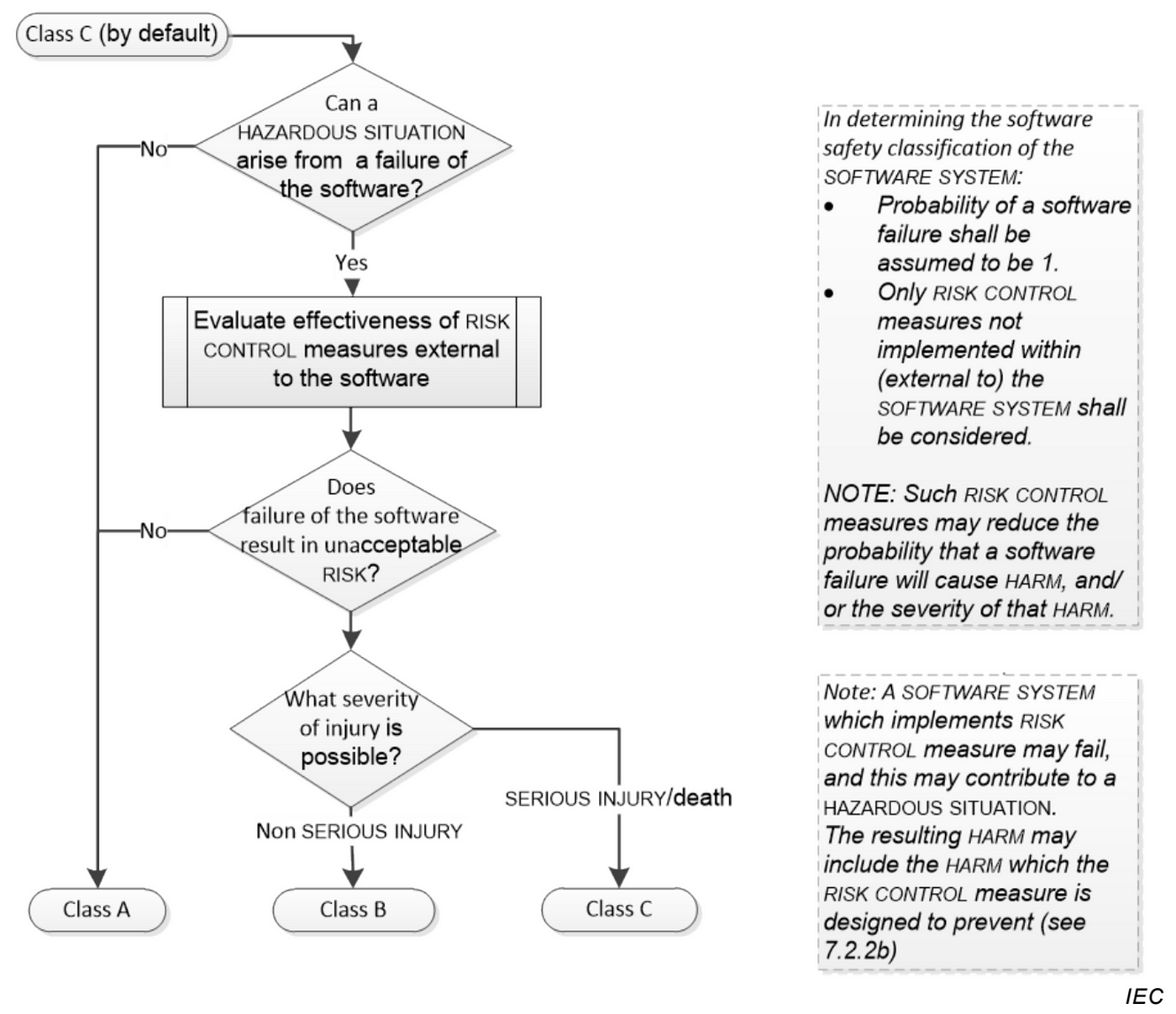
IEC 62304: Medical Device Software LifeCycle Processes | by Dr Stephen Odaibo | The Blog of RETINA-AI Health, Inc. | Medium